IMMOBILIZATION DEVICE FOR PATIENTS UNDERGOING CRANIO-SPINAL IRRADIATION
Gunilla C. Bentel, RN, RTT
Duke University Medical Center
Durham, North Carolina
INTRODUCTION
Cranio-spinal irradiation (CSI) is required in patients with medulloblastoma, and sometimes in patients with lymphoma and leukemia, usually as a preventive mode of therapy. Patients requiring CSI are usually children or young adults since these tumors most frequently occur in the relatively young population. The technique applied in the treatment of the CSI usually require that the patient is in the prone position while the brain and cervical spine is treated through opposed lateral fields and the spinal axis through one or two posterior fields.
For most patients, the prone is less comfortable than supine and is therefore less likely to be maintained throughout a treatment session unless they are positioned in a body shell. Children often move unintentionally and if they are in an uncomfortable position the risk of movement is increased. Comfortable and effective immobilization techniques are therefore needed.
TREATMENT
The most commonly used treatment technique for CSI consists of opposed lateral brain and cervical spine fields and one or two posterior spinal fields. To avoid beam overlap at the junction between the lateral and the posterior fields, the couch is turned such that the caudal margin of the lateral brain field traverse the neck perpendicular to the spin axis.
The collimator is turned until the caudal margin of the brain fields is parallel with the diverging posterior field margin. This field matching technique is similar to that frequently used when matching multiple breast treatment fields. The proximity of the lens of the eye to the radiation beam and the difficulties with matching of these field margins require an effective immobilization device.
For nearly 15 years, customized half-body plaster casts have been made in our department for patients requiring CSI. These casts were relatively easy to make but required 2 days to dry before they became strong enough to support the patient’s weight. These casts, because they also included the arms, were often wider than the couch. To enter and exit the casts in the prone position, it was necessary for the patients to brace themselves with both arms on the cast. The pressure thus exerted on the cast was often more than the cast could tolerate. For many patients, particularly those with neurologic deficits, getting in and out of the cast was difficult. Another problem was the delay by 2 to 3 days in starting the treatments because the cast needed to dry before it was strong enough to support the patient’s weight. An increasing demand to begin treatment with short notice resulted in the development of a CNS ALPHA CRADLE® device for patients receiving CSI.
POSITIONING DEVICE
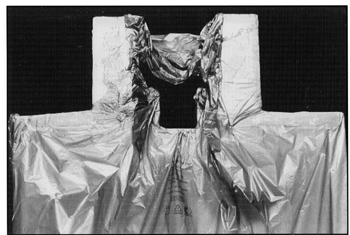
The CNS ALPHA CRADLE® device, consists of a Styrofoam®* form which roughly fits the shape of the prone patient’s head and torso. The head form, which is attached to the body form, consists of four walls that are approximately 4” high (Fig. 1). These walls support the head and has an opening for the patient’s face. A 1.5 mil polyvinyl chloride (PVC) sheet is taped to the edge of the four walls so that is forms a channel approximately 2” in diameter. The channel opens into the body form on each side of the head. The body form is enclosed in a PVC sheet to prevent any foam from touching the patient. The head support is placed so that the patient’s head is partially over the edge of the couch provide unobstructed view and air flow while the CNS ALPHA CRADLE® device is being made.
Approximately 30 cc of foaming agents are poured into each opening of the channel and about 600 cc is poured into the body form before the patient lies down in the prone position. The patient’s head is resting on the channel where the foam has been poured. The foam swells up around the patient’s face leaving an opening
*Registered by Dow Chemical
for the eyes, nose and mouth. The PVC sheet covering the body part of the CNS ALPHA CRADLE® device is pulled up between the patient’s arms and the torso so that the foam can raise and form a mold of the arms as well as of the torso (Figs. 2A and 2B).
When the foam has hardened, the patient lifts up the head and a softened thermoplastic sheet is placed across the head support. The patient then resumes the position and thermoplastic sheet is pressed up against the face to form a tight mask (Fig 2B). When the patient lies down, a visual check is made to see that the patient is straight and that the chin is raised so that the posterior spinal field will not exit through the oral cavity. In some patients it is necessary to elevate the torso so that the head and the torso are level. This can be accomplished by placing a large piece of Styrofoam® in the body part of the CNS ALPHA CRADLE® form prior to pouring the foaming agents.
During the simulation procedure, openings are made in the Styrofoam® and the thermoplastic sheet so that the patient’s eyes can be visualized. Markers are placed on the lateral canthus of the eyes during the simulation procedure and a visual check is made to assure that the lens of the eye is out of the radiation field during each treatment set-up.
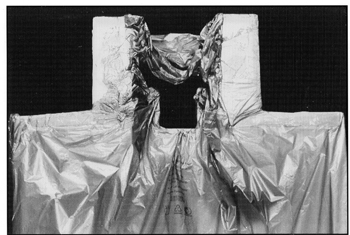
Fig. 1 A polyvinyl sheet is taped around the face support to form a channel around the head. The foam will expand inside the polyvinyl sheet and form a face support. A polyvinyl sheet is also taped over the torso portion of the Alpha Cradle® form. Foaming agents poured into this section will expand and form a mold around the patient’s torso.
RESULTS
The CNS ALPHA CRADLE® device, is fairly new and has successfully been used in patients. One was and adult patient with hemiparalysis. He was able to get in and out of the CNS ALPHA CRADLE® device with very little help. It was felt by the technologists who treated the patient, that if he had been in a total body plaster cast, it would have required several people to help him get in and out of the cast. Review of port films of the patients treated in this CNS ALPHA CRADLE® device indicated that the repositioning was excellent.
DISCUSSION
Precise repositioning of patients in the prone position is quite difficult and for many patients it is also a difficult position to maintain. Patients requiring CSI often present with some neurological deficits that makes positioning even more difficult. Complex geometric matching of beam edges in the spinal cord and the proximity of critical organs requires precise repositioning of the patient for each treatment session. This CNS ALPHA CRADLE device has proven to be effective, easy to make, and comfortable for the patients.
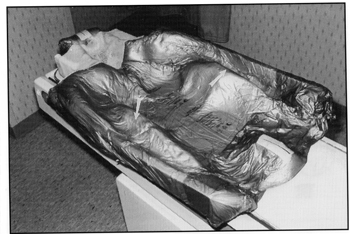
Fig. 2A An impression of the arm position, made by pulling the polyvinyl sheet between the arms and the torso, aids in repositioning the arms.
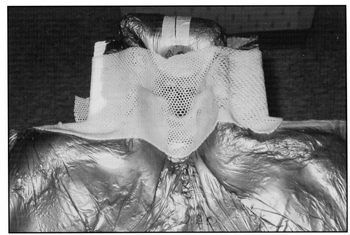
Fig. 2B A thermoplastic sheet, softened in warm water and draped over the patient’s face, aids in repositioning the head.